-
 Figure 1: Charge variants of IgG4 based monoclonal antibodies can be successfully evaluated by AEX-MS.
Figure 1: Charge variants of IgG4 based monoclonal antibodies can be successfully evaluated by AEX-MS. -
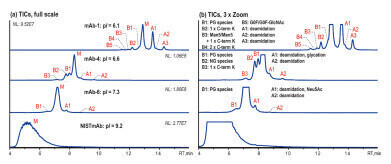 Figure 2: Native AEX-MS charge variant analysis of three different IgG4-based mAbs and NISTmAb, shown basic (B) and acidic (A) variant peaks as well as the main species (M), 5 μg injection [2].
Figure 2: Native AEX-MS charge variant analysis of three different IgG4-based mAbs and NISTmAb, shown basic (B) and acidic (A) variant peaks as well as the main species (M), 5 μg injection [2]. -
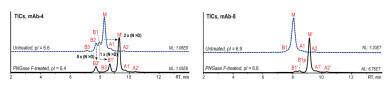 Figure 3: Native AEX-MS analysis of mAb-4 (left) and mAb-8 (right) before (blue) and after PNGase F-treatment (black) [2].
Figure 3: Native AEX-MS analysis of mAb-4 (left) and mAb-8 (right) before (blue) and after PNGase F-treatment (black) [2].
Bioanalytical
Charge variant AEX-MS analysis of IgG4-based mAbs!
Nov 21 2023
Cation exchange chromatography (CEX) is an excellent method of characterising the charge heterogeneity of biomolecules. Most commercially available monoclonal antibodies (mAbs) are often IgG1-based and possess a high isoelectric point (pI) of usually ≥8. Therefore, CEX, optionally coupled with mass spectrometry (MS), is the conventional approach [1]. However, for IgG4-based mAbs, which possess a pI<8, anion exchange chromatography (AEX) may be an alternative approach.
This technical note describes the successful application of an AEX method for charge heterogeneity analysis of IgG4-based mAbs coupled to a MS detector. Several IgG4-based mAbs with different pIs as well as the NISTmAb were analysed using a BioPro IEX QF anion exchange column [2].
Native AEX-MS method development
A salt gradient from 10 mM ammonium acetate (A) to 300 mM ammonium acetate (B) (pH unadjusted) is used to analyse various IgG4-based mAbs (pI 6.1–7.3) and the NISTmAb, which is IgG1-based and has a pI of 9.2. Figure 2 shows that the AEX-MS method is suitable for IgG4-based mAbs with moderate pIs, but not for IgG1-based mAb with higher pI. The separation improves as the pI gets lower.
The acidic variants separated correlate with those commonly observed by CEX-MS such as deamidation, glycation and sialic acid (Neu5Ac)-containing species. The basic variants observed can be identified as unprocessed C-terminal Lys (C-term K) and mAb species with different numbers of Fc N-glycans. In addition, the glycoforms Man5/Man5 with unprocessed C-terminal K and G0F/G0F-GlcNac are observed for mAb-1. mAb-4 demonstrates that this AEX-MS method is very sensitive to the macroheterogeneity of Fc N-glycosylation. The main fully glycosylated (FG) species is separated from the partially glycosylated (PG) peak B1 and the non-glycosylated (NG) species B2, which elute earlier.
Further improvement of the basic variant separation
Since mAbs with a lower pI are better separated, it was tested to see whether the separation can be improved by lowering the pI through PNGase F-mediated deglycosylation. This reaction removes N-glycans and simultaneously converts the glycan-bearing asparagine (Asn) residue to aspartic acid (Asp). Since all IgG4 mAbs contain an Asn residue in the Fc region of each of the two heavy chains, up to two Asn to Asp conversions can be expected.
Figure 3 shows the differences between the untreated and the PNGase F-treated mAbs mAb-4 (pI=6.6) and mAb-8 (pI=6.9). The PNGase F-treatment decreased the pI to 6.4 for mAb-4 and 6.6 for mAb-8. Due to the reduced pI the overall retention as well as the variant separation and peak sharpness are improved for both mAbs.
Summary
AEX-MS analysis is very suitable for characterising charge heterogeneity in IgG4-based mAbs. The resolution of the glycosylated variants can be further improved by PNGase F-mediated deglycosylation.
The technical note further demonstrates that AEX-MS provides overall better separation of the IgG4-based mAb with moderate pI and provides additional information compared to CEX-MS. Furthermore, the subunit analysis of IgG4-based mAbs after digestion with IdES protease is discussed. To monitor critical FC quality attributes the improved resolution of a PNGase F-treatment of the fragments was utilised.
Literature
[1] Y. Yan, A. P. Liu, S. Wang, T. J. Daly, N. Li, Ultrasensitive Characterization of Charge Heterogeneity of Therapeutic Monoclonal Antibodies Using Strong Cation Exchange Chromatography Coupled to Native Mass Spectrometry, Anal. Chem. 2018, 90, 13013-20.
[2] A. P. Liu, Y. Yan, S. Wang, N. Li, Coupling Anion Exchange Chromatography with Native Mass Spectrometry for Charge Heterogeneity Characterization of Monoclonal Antibodies, Anal. Chem. 2022, 94, 6355−62.
Digital Edition
Chromatography Today - Buyers' Guide 2022
October 2023
In This Edition Modern & Practical Applications - Accelerating ADC Development with Mass Spectrometry - Implementing High-Resolution Ion Mobility into Peptide Mapping Workflows Chromatogr...
View all digital editions
Events
Apr 28 2024 Montreal, Quebec, Canada
May 05 2024 Seville, Spain
May 15 2024 Birmingham, UK
May 19 2024 Brno, Czech Republic
May 21 2024 Lagos, Nigeria