-
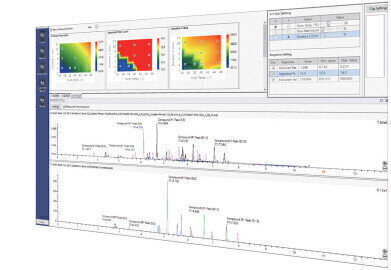 LabSolutions MD Software Window.
LabSolutions MD Software Window. -

Chromatography
New Method Development Solution: Software Support for Analytical HPLC Method Development and Optimisation
Jan 13 2022
Shimadzu, one of the world leaders in analytical instrumentation, has announced the release of its ‘LabSolutions MD’ software for analytical method development for high-performance liquid chromatography (HPLC). In addition to established ‘Method Scouting’ functionality that enables automated, quick and simple column and solvent screening, the new LabSolutions MD method development solution supports ‘Analytical Quality by Design’ (AQbD) as proposed by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), to emphasise scientific foundations and risk assessment for newly developed analytical methods.
LabSolutions MD software supports method development according to AQbD principles. In combination with the Nexera UHPLC Method Scouting System, it offers a graphical user interface to simplify the system setup, as well as automated batch creation, to provide reliable data while minimising the risk of human error. It enables screening of various combinations of different mobile phases and separation columns to rapidly identify a suitable combination visually and quantitatively according to specified parameters, such as number of separated peaks and resolution. With the selected combination, LabSolutions MD can then create a multifactorial experimental design, where using only limited input data, the software is able to determine optimum separation conditions within the design space. Accurate retention modelling allows an estimation of method robustness in minimal time.
LabSolutions MD, in combination with the Shimadzu LabSolutions chromatography data system (CDS), provides data management, statistical evaluation and intuitive reporting, all in a LabSolutions database, ensuring data integrity.
LabSolutions MD key features
LabSolutions MD is seamlessly integrated in the LabSolutions CDS and features the same graphical user interface as the established Method Scouting Solution software, ensuring a quick and easy system setup. In addition to fully automated method screening, it offers:
Rapid Identification of Optimum Separation Conditions using Design of Experiments (DoE)
Using the ‘trial-and-error’ approach, a large number of experiments can be necessary to establish a suitable separation method, especially with complex samples. With a multifactorial experimental design, only a limited amount of experimental data is required to create a defined design space. Computer simulation and retention modelling within this design space allow for fast, statistically informed decisions on suitable separation conditions, while reducing the risk of human error.
Visualisation of Optimum Separation Conditions in a Resolution Map
The effect of changes in analytical conditions on chromatographic resolution within the design space is visualised in a color-coded map that enables effortless identification of robust method conditions, where a separation is less likely to be affected by small variations in method parameters. Computer simulation can be used to create a virtual chromatogram view of analysis with the specified conditions.
Integrated Database Management of the Analytical Method Development Process
LabSolutions CDS creates summary reports that include all collected data. In addition, the software can provide integrated management of the entire sequence of files in a database to ensure data integrity.
Strict quality control is required for new and established drug products during development as well as production. HPLC and UHPLC are most often the methods of choice in pharmaceutical QA/QC departments. To ensure patient safety, development of accurate, highly reliable analytical methods is a pre-requisite for pharmaceutical manufacturers. Using a ‘trial-and-error’ approach often leads to less than optimum separation conditions and little robustness against small changes in one or two method parameters. Also, it can be time consuming, even for experienced scientists with the necessary expertise.
AQbD promotes comprehensive evaluation of the analytical conditions, without relying on an operator’s experience or intuition. It increases reliability and enables the efficient development of accurate, robust analytical methods.
More information online
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
Apr 22 2024 Marrakech, Morroco
Making Pharmaceuticals Exhibition & Conference
Apr 23 2024 Coventry, UK
Apr 23 2024 Kintex, South Korea
Apr 23 2024 Seoul, South Korea
Apr 24 2024 Jakarta, Indonesia







.jpg)